A 2.5 g sample of french fries is placed in a calorimeter with 500.0 g of water at an initial temperature of 21 °C. After combustion of the french fries, the water has a temperature of 48 °C. What is the energy value (kJ/g) of french fries? A) 100 kJ/g B) 40 kJ/g C) 0.14 kJ/g D) 56 kJ/g E) 23 kJ/g
Recipe This | Ninja Foodi Frozen French Fries
Aug 30, 2023answer answered a 2.5 g sample of french fries is placed in a calorimeter with 500z0g of water at an intial temperture of 21 degrees celcius agter combustion of the frnech fries the water has a temperature of 48 degrees celcius. what us rhe caloric calue (kcal/g) of the french fries Advertisement shadayrobinson2000 is waiting for your help.

Source Image: constantlycooking.com
Download Image
A 2.5 g sample of french fries is placed in a calorimeter with 500.0 g of water at an initial temperature of 21 °C. After combustion of the french fries, the water has a temperature of 48 °C. What is the caloric value (kcal/g) of the french fries?
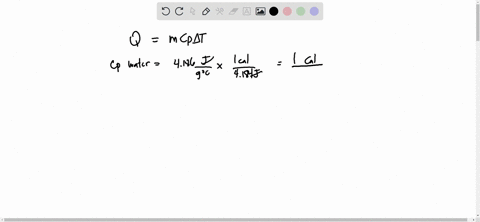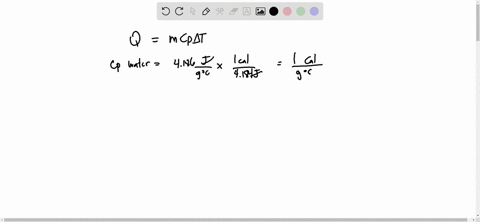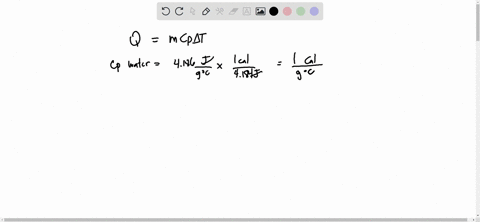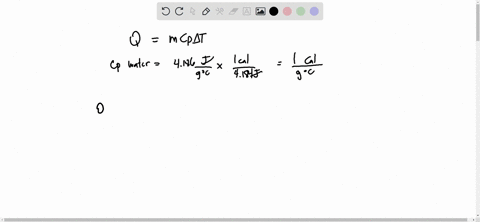
Source Image: numerade.com
Download Image
Straight Cut 7*7mm / 9*9mm Frozen French Fries in 2.5kgs Bag Packages – China Frozen Food, French Fries | Made-in-China.com
Verified answer A 2.5 g sample of french fries is placed in a calorimeter with 500.0 g of water at an initial temperature of 21 °C. After combustion of the french fries, the water has a temperature of 48 °C.
Source Image: mdpi.com
Download Image
A 2.5 G Sample Of French Fries
Verified answer A 2.5 g sample of french fries is placed in a calorimeter with 500.0 g of water at an initial temperature of 21 °C. After combustion of the french fries, the water has a temperature of 48 °C.
Expert Answer 100% (1 rating) Transcribed image text: A 2.5 g sample of french fries is placed in a calorimeter with 500.0 g of water at an initial temperature of 21°C. After combustion of the french fries the water has a temperature of 48°C. What is the caloric value (kcal/g) of the french fries?
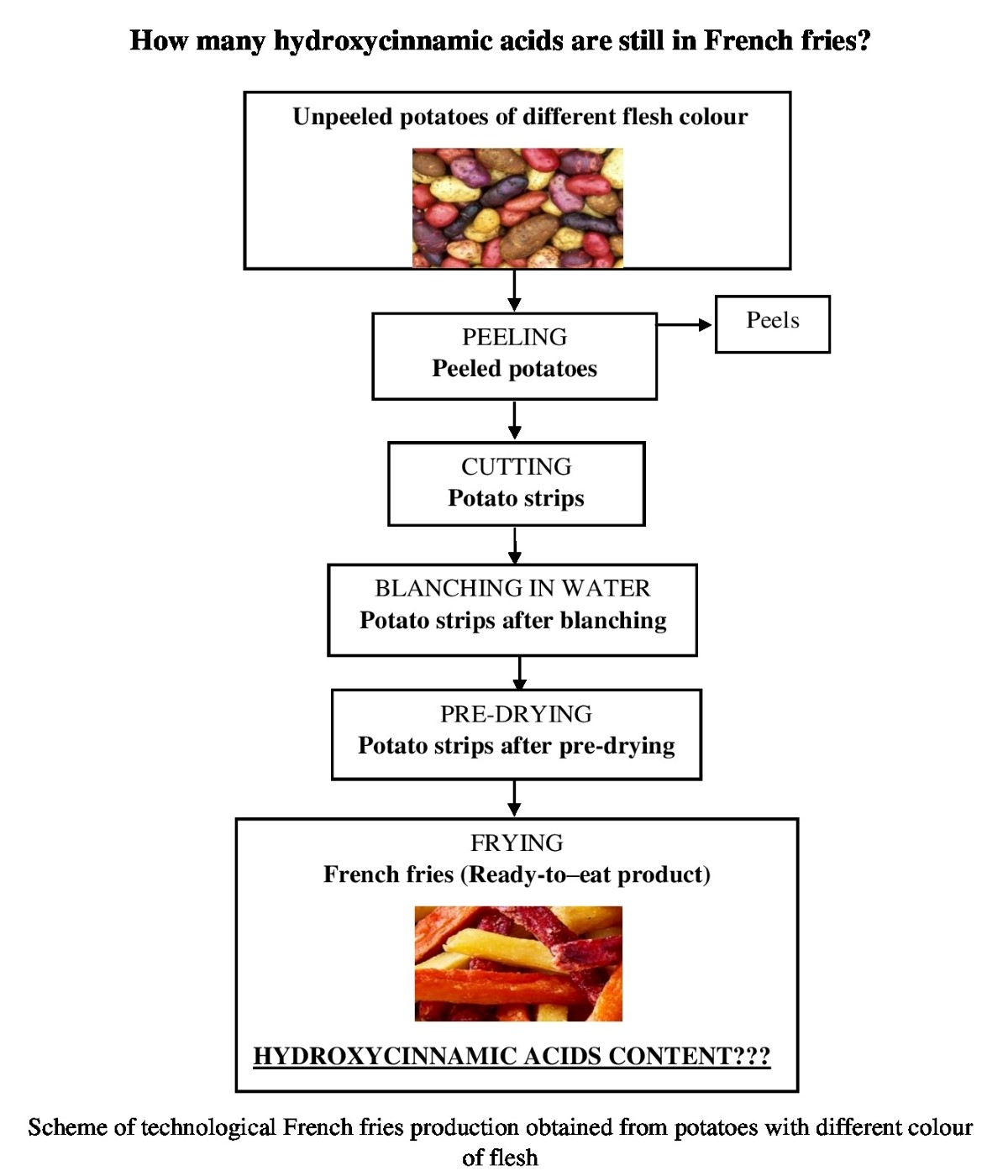
Antioxidants | Free Full-Text | Content and Stability of Hydroxycinnamic Acids during the Production of French Fries Obtained from Potatoes of Varieties with Light-Yellow, Red and Purple Flesh
A 2.5 g sample of french fries is placed in a calorimeter with 400 g of water at an initial temperature of 20°C. After the combustion of the french fries, the water has a temperature of 50°C. What is the caloric value (kcal/g) of the french fries? Solution Verified Answered 7 months ago Create an account to view solutions
Foods | Free Full-Text | Instrumentation for Routine Analysis of Acrylamide in French Fries: Assessing Limitations for Adoption

Source Image: mdpi.com
Download Image
HACCP Standard Crispy and Less Salt Frozen French Fries in 2.5 Kgs/ Bag – China Frozen French Fries, French Fries HACCP | Made-in-China.com
A 2.5 g sample of french fries is placed in a calorimeter with 400 g of water at an initial temperature of 20°C. After the combustion of the french fries, the water has a temperature of 50°C. What is the caloric value (kcal/g) of the french fries? Solution Verified Answered 7 months ago Create an account to view solutions

Source Image: m.made-in-china.com
Download Image
Recipe This | Ninja Foodi Frozen French Fries
A 2.5 g sample of french fries is placed in a calorimeter with 500.0 g of water at an initial temperature of 21 °C. After combustion of the french fries, the water has a temperature of 48 °C. What is the energy value (kJ/g) of french fries? A) 100 kJ/g B) 40 kJ/g C) 0.14 kJ/g D) 56 kJ/g E) 23 kJ/g

Source Image: recipethis.com
Download Image
Straight Cut 7*7mm / 9*9mm Frozen French Fries in 2.5kgs Bag Packages – China Frozen Food, French Fries | Made-in-China.com
A 2.5 g sample of french fries is placed in a calorimeter with 500.0 g of water at an initial temperature of 21 °C. After combustion of the french fries, the water has a temperature of 48 °C. What is the caloric value (kcal/g) of the french fries?

Source Image: qdkeqing.en.made-in-china.com
Download Image
Kenji Knows French Fries. Our students get science-y with Kenji… | by SF Cooking School | SF Cooking | Medium
A 2.5 g sample of french fries is placed in a calorimeter with 500.0 mL of water. The initial temperature of the water is 21.0 0 C. After combustion of the french fries, the water has a temperature of 48.0 0 C. Calculate the heat gained by the water (calories). Remember–the density of water is 1.00 g/mL.

Source Image: medium.com
Download Image
Seaweed chips (baked) with miso mayo – Lazy Cat Kitchen
Verified answer A 2.5 g sample of french fries is placed in a calorimeter with 500.0 g of water at an initial temperature of 21 °C. After combustion of the french fries, the water has a temperature of 48 °C.

Source Image: lazycatkitchen.com
Download Image
French Fries – www.foodexeg.com
Expert Answer 100% (1 rating) Transcribed image text: A 2.5 g sample of french fries is placed in a calorimeter with 500.0 g of water at an initial temperature of 21°C. After combustion of the french fries the water has a temperature of 48°C. What is the caloric value (kcal/g) of the french fries?

Source Image: foodexeg.com
Download Image
HACCP Standard Crispy and Less Salt Frozen French Fries in 2.5 Kgs/ Bag – China Frozen French Fries, French Fries HACCP | Made-in-China.com
French Fries – www.foodexeg.com
Aug 30, 2023answer answered a 2.5 g sample of french fries is placed in a calorimeter with 500z0g of water at an intial temperture of 21 degrees celcius agter combustion of the frnech fries the water has a temperature of 48 degrees celcius. what us rhe caloric calue (kcal/g) of the french fries Advertisement shadayrobinson2000 is waiting for your help.
Straight Cut 7*7mm / 9*9mm Frozen French Fries in 2.5kgs Bag Packages – China Frozen Food, French Fries | Made-in-China.com Seaweed chips (baked) with miso mayo – Lazy Cat Kitchen
A 2.5 g sample of french fries is placed in a calorimeter with 500.0 mL of water. The initial temperature of the water is 21.0 0 C. After combustion of the french fries, the water has a temperature of 48.0 0 C. Calculate the heat gained by the water (calories). Remember–the density of water is 1.00 g/mL.